Difference between green and blue hydrogen
The importance of the colours of hydrogen
Hydrogen is the most abundant chemical element in the universe, but it is not found in free form, so it has to be produced. It is what is known as an energy vector, i.e., it has the capacity to store energy that will later be released. From hydrogen we can obtain electrical, mechanical or thermal energy without CO2 emissions and with high performance, as well as chemical products with high added value such as ammonia, methanol, etc. In the different processes mentioned above, the fundamental residue of the processes is pure water.
The point is that if we want to use it for electricity production, a key move in contributing to the energy transition, we need to extract it from common compounds on our planet, such as water, biomass or waste.
Depending on the process used to extract the hydrogen, we can speak of a renewable or non-renewable source. To understand this at a glance, the hydrogen colour code has been created.
The colours of hydrogen indicate, at a glance, both the extraction process followed and the source of energy used, as well as the compound from which we start. Thus, we have from green hydrogen (obtained from renewable energy sources) to grey or black hydrogen (those with the most polluting processes). In this article we will look at the differences between green and blue hydrogen, which are two of the best-known colours.
Blue hydrogen
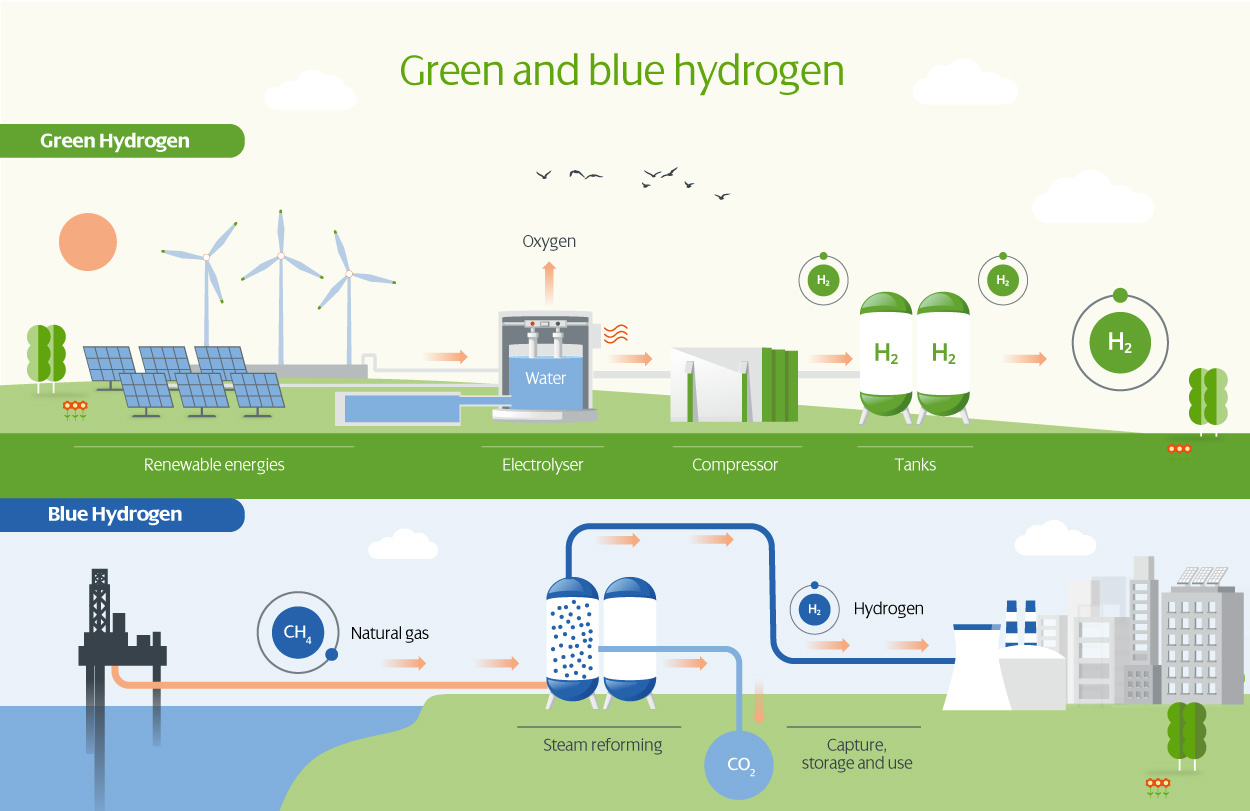
The process for obtaining blue hydrogen involves hydrocarbons. From compounds such as methane, for example, a chemical process called "reforming" is carried out to obtain hydrogen on the one hand, and carbon dioxide on the other.
This CO2 can be captured, released into the atmosphere or use. In the blue hydrogen process, they use CO2 capture systems from reforming technology but they have an efficiency of 60-65%, so there is 30-35% of carbon dioxide that will be emitted.

Green hydrogen
An alternative that reduces emissions and cares for our planet.

Hydrogen stations
What are hydrogen stations and how do they work?

Green ammonia
The sustainable revolution in the chemical industry.

Green methanol and the maritime transport
This chemical compound is a promising alternative to fossil fuels.
Green hydrogen
Green hydrogen is obtained by separating hydrogen from oxygen through the electrolysis of water. This electrolysis can be carried out with energy from renewable sources, which makes the process more sustainable and provides us with a clean energy source. The hydrogen obtained in this way can be stored or used in industrial or heavy mobility processes, while the resulting oxygen is released into the atmosphere or can be used as a by-product. In the same way, the electrolyser generates heat that can be harnessed through the use of heat pump technologies for district heating. It is therefore a pollutant-free process.
Despite the high cost of this type of energy, Iberdrola is committed to it, as demonstrated by the Puertollano plant and various projects to replace grey hydrogen with green hydrogen, as well as the generation of hydrogen derivatives such as ammonia or methanol.
Differences between blue and green hydrogen
The main difference between green and blue hydrogen lies in the process of obtaining the hydrogen, and in its environmental impact. Blue hydrogen does not reduce energy dependence on gas and perpetuates a development model based on fossil fuels.
In the case of blue hydrogen, the main problem is the carbon dioxide obtained from the reforming of natural gas (whit an efficiency of 60-65%), which has to be captured and stored. Moreover, the use of hydrocarbons means that renewable sources are not used, and emissions are not zero.
- Green hydrogen: 0 kgCO2 /kg H2.
- Blue hydrogen: 3.5-4 kgCO2 /kg H2.
- Grey hydrogen: 10 kgCO2 /kg H2.
Green hydrogen, however, is totally clean and is obtained from a renewable resource, using green energy sources.
Another relevant aspect is water consumption. To generate 1 kg of hydrogen from electrolysis requires 10 litres of deionised water; from natural gas reforming with CO2 capture requires 25 litres of deionised water and from natural gas reforming without CO2 reforming requires 23.5 litres. This means that generating green hydrogen from electrolysis technology is more efficient from the point of view of water, one of the essential raw materials along with renewable electricity.
Another difference is economic. Blue hydrogen has a lower initial production cost due to the use of hydrocarbons, among other reasons; green hydrogen has a higher production cost for the time being, but the development of new green hydrogen plants and the convinced commitment to the generation of this element means that, little by little, the difference in terms of cost is beginning to fade.
The last difference is strategic, as green hydrogen favours self-sufficiency by being able to use existing natural resources from all over the world, which limits or eliminates dependence on third countries. Blue hydrogen has the disadvantage of being dependent on natural gas producing countries.
The other colours of hydrogen
In addition, other hydrogen colours are defined. The most common is grey hydrogen, with an extraction process very similar to blue hydrogen, but in this case, carbon dioxide emissions are not controlled and pollution is higher.
Pink hydrogen uses electricity generated in a nuclear power plant for electrolysis, while yellow hydrogen is hydrogen where the electricity used for electrolysis comes from mixed sources, ranging from renewable energy to fossil fuels. In particular, green hydrogen obtained from solar energy is considered yellow hydrogen. Other more minor and polluting colours would be turquoise (from methane pyrolysis) or black (obtained from coal combustion).