Green methanol
Green methanol: the fuel that can accelerate the energy transition in shipping
Green methanol is methanol that is produced renewably and without polluting emissions, one of its variants being generated from green hydrogen. This chemical compound can be used as a low-carbon liquid fuel and is a promising alternative to fossil fuels in areas where decarbonisation is a major challenge, such as maritime transport.
What is green methanol?
Methanol (CH₃OH), also known as methyl alcohol, is a chemical compound in great demand due to its many industrial applications: as a solvent, antifreeze, in building materials and in the production of synthetic fuels, among others. As a light liquid at room temperature, it is easy to transport and store. To date, the vast majority of methanol produced is generated from natural gas, which links it directly to greenhouse gas emissions. The advent of green methanol from clean energy sources is intended to give a major boost to this compound, making it a promising alternative to fossil fuels in reducing the carbon footprint of high emitting industries such as maritime transport.
Grey, blue and green methanol
Like other compounds or materials, methanol is nowadays classified according to the degree of sustainability of its production process, making it a more or less environmentally friendly raw material, and therefore more or less useful for contributing to decarbonisation. Thus, we must clearly distinguish green methanol from blue or grey methanol.
Grey methanol
It is obtained by synthesis reaction from methane present in natural gas (or in some cases, as in China, still from coal). It is therefore not a renewable or clean energy.
Blue methanol
It is also obtained by synthesis derived from natural gas, but includes as part of the process the capture and storage of the carbon generated during its production, converting it into a less polluting product.
Green methanol
It is produced using only renewable energy sources in the process and ensuring that no harmful gases are emitted into the atmosphere. Green methanol is thus synonymous with clean, renewable methanol.
-
Biomethanol: produced from the gasification(1) of sustainable biomass sources such as livestock, agricultural and forestry residues and municipal waste.
- e-methanol: produced from hydrogen produced from renewable electricity (what we call green hydrogen) and captured carbon dioxide.
(1) Combustion at a temperature between 700 and 1,500 ºC.

Green hydrogen
An alternative that reduces emissions and cares for our planet.

Hydrogen stations
What are hydrogen stations and how do they work?

Difference between green and blue hydrogen
Discover the importance of the colours of hydrogen.

Green ammonia
The sustainable revolution in the chemical industry.
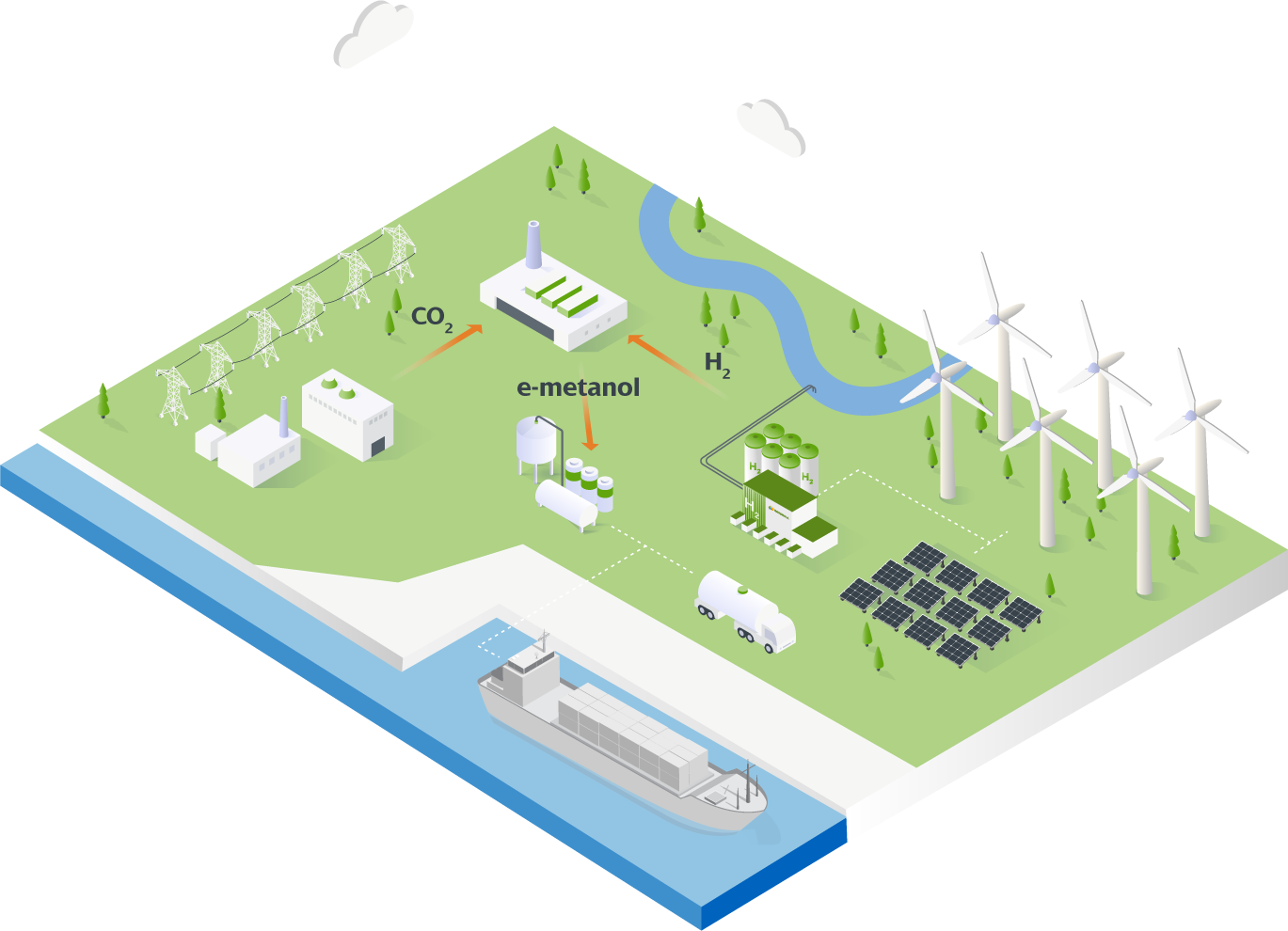
From green hydrogen to green methanol
Green hydrogen is present on the roadmap of any of the economic and political actors responsible for tackling the current energy crisis and facilitating compliance with the zero emissions targets set for 2050. At Iberdrola, we have been anticipating and committing to the development of this energy as a key element of decarbonisation for years.
Now, the interest in green methanol production opens up a new growth opportunity for the green hydrogen sector for e-methanol generation.
Green hydrogen is generated through a chemical process known as electrolysis, which uses electric current to separate hydrogen from the oxygen in water. When this electricity is obtained from renewable sources, such as a wind or photovoltaic farm, we will produce energy without emitting carbon dioxide into the atmosphere. If the hydrogen from these renewable sources is synthesised together with the captured carbon dioxide - in a biomass power plant after the pyrolysis process(2) - renewable methanol or e-methanol can be distilled.
(2) Biomass decomposition in an oxygen-free environment with a temperature of approximately 500°C.
 SEE INFOGRAPHIC: How green methanol is produced from green hydrogen [PDF]
SEE INFOGRAPHIC: How green methanol is produced from green hydrogen [PDF]
Uses of green methanol
The expansion of green methanol as an alternative fuel to fossil fuels is particularly attractive to the maritime industry because, being liquid at room temperature, it is much less costly to store and transport than gaseous fuels, and has the lowest carbon footprint of all liquid fuels. It can also be used in both internal propulsion engines and to power fuel cells, providing flexibility depending on individual needs.
The trend is clear. As large multinationals such as the Danish shipping company Maersk sign commitments to produce green hydrogen ad hoc, for the generation of e-methanol to decarbonise their freight transport fleet, at Iberdrola we are already part of the alliance to lead the production of green methanol in Spain together with the company Foresa.
Our Green Umia project, with a budget of €40 million, will locate a green methanol plant in the Galician town of Caldas de Reis, 12 kilometres from the Castro Valente wind farm with which it has an energy supply agreement to guarantee the green consideration. Green Umia estimates to achieve a reduction of 58,000 tonnes of CO2 during its first 10 years of operation, as a result of the production of 2,900 tonnes per year of renewable methanol, which will be used by Foresa itself for incorporation into a wide variety of industries.